By November 2018, the first set of the European Standards of Care for Newborn Health (ESCNH) were developed by about 220 experts from all over Europe.
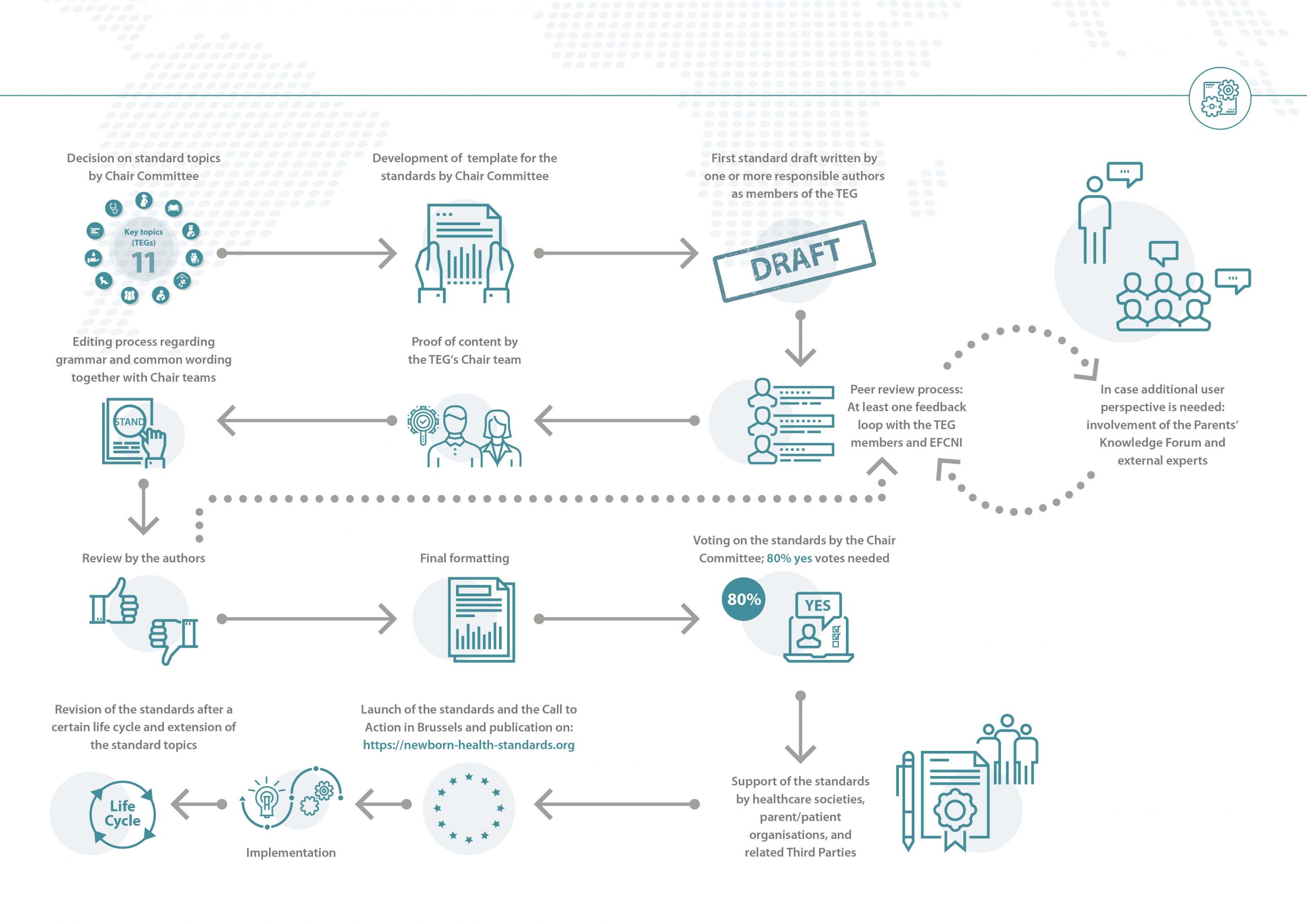
Click on the graphic below to receive an overview on the development process for the standards, starting with the decision on the standard topics, ending with their official launch and beyond.
Within the European Standards of Care for Newborn Health (ESCNH), a single standard is defined as a systematically developed statement with the purpose to support decision making of physicians, nurses, and patients for adequate care regarding specific health problems. These standards serve as a benchmark and blueprint for the development of standards in each individual country.
The ESCNH are reference standards that need to be translated into national binding guidelines, standards, recommendations, directives, or laws (depending on the respective national situations).
The overall aim of the ESCNH is to ensure that across Europe, the care for preterm and ill babies is harmonised and conform with current best available evidence.
In a first step the overarching topics of the ESCNH are decided by the Chair Committee, the project’s steering committee and decision-making body. After the development of the standard template by the Chair Committee, the members of the interdisciplinary Topic Expert Groups (TEGs) work towards the first standard drafts for their topics. In every TEG, aspects of neonatal care in need of a universal standard are identified. Then one or more responsible authors develop and draft a corresponding standard. This is followed by a peer review process during which the standard drafts pass through at least one feedback loop with all other TEG members. The TEG’s exchange during this period happens virtually via telephone and video conferences or via email.
A further supporting body on request is the Parents’ Knowledge Forum – represented by EFCNI’s worldwide parent and patient organisations network – which can be consulted for additional user perspectives on specific questions.
Before the standards enter the editing process, the chairs, two to four people responsible for all standards in the their respective TEG, review the proposed standard texts and check the content for correctness. Once approved by the chairs of the TEG, each standard is revised by the editorial team to ensure a common wording as well as a similar structure. This version is passed back to the authors of the respective standards for approval. In case the authors do not approve the edits or in case there are any queries that require adaptation of the standard, the feedback loop with the whole TEG starts again.
The approved final version then passes a last formatting process by EFCNI’s design department.
Each individual standard has to be voted on electronically by the Chair Committee. There are three options: “yes”, “no”, and “abstain”. When voting “no” or “abstain”, one has to give a reason for this decision, which will be passed on to the authors and chairs of the respective standard so that they can revise the standard in question. The acceptance and publication of a standard requires an 80 percent majority of all “yes” and “no” votes (the voting option “abstain” is not counted in).
As a first step of endorsement, all standards are sent to European and national healthcare societies and organisations, as well as parent and patient organisations to receive their official support for the newly developed standards. All supporting organisations can be found here.
The official launch of the ESCNH took place in the European Parliament in Brussels on 28 November 2018. From that day on, the standards have been available for download here. Furthermore, the launch event was accompanied by a Call to Action for Newborn Health to make decision makers aware of their responsibilities in regards to newborn health.
More information on the launch event, the agenda as well as a short summary are available here.
As medical care is a fast-developing area, the ESCNH need to be revised regularly. Therefore, the chairs together with the author group have determined a certain interval (3, 5, or 10 years) for each standard after which it needs to be revised. Of course, if the authors or the Chair Committee see the need to revise a standard in-between a lifecycle interval, an earlier revision is possible. New standards will continuously be developed during the next years, following the same methodology to enlarge the ESCNH package.
Starting from November 2018 onwards, each standard is thus valid for a certain time interval (called lifecycle). Whenever a standard’s lifecycle nears completion, EFCNI contacts the respective chairs and authors for a review and decision on what needs to be adapted because of new developments that may have occurred during the interval. Chairs and authors will have approximately one year (revision period), during which they can update the standard document. To ease the revision process, every standard is reviewed by an independent expert (expert consultation) and other stakeholders as well as the greater community (public consultation). More information on the consultation process can be found here. The revised standards will then again be voted on by the Chair Committee. After the voting, the lifecycle will restart.
To facilitate a harmonised format and structure of the standards, a specific template was created by the Chair Committee for the development process.
The most important part of the template is the component table, which displays the standard per se, separated in a section for parents and family, for healthcare professionals, for hospitals, and for health services. These sections are adapted according to the needs of the respective parties involved in a certain area of care. The single components are graded in accordance with their level of evidence (see below) and indicators to evaluate the standard are included for each component (see below).
Two further tables display potential future developments of care, in case a hospital already meets all core components of the standard, and initial steps for those hospitals where the components or some of the components seem to be well beyond the current levels of care.
Please note that the standards are kept relatively short to make them applicable to individual settings in the respective country. For example, resources such as money, personnel, rooms, time, equipment, etc. are not explicitly mentioned in every standard as they strongly depend on the local situation. They have to be identified in preparation of the local standard implementation.
Click on the graphic below for explanations on every section of the standards template.

The tables are each separated into a section for parents and family, for healthcare professionals, for the neonatal unit, for the hospital, and the health service. If applicable, the sections could be combined or adapted to the respective standard topic.
Notably, if a neonatal unit is mentioned, the standard does not only refer to infants up to the age of 6 weeks but to older infants as well. In countries where infants are then transferred to another unit (e.g. paediatric unit) or where neonatal units do not exist, the standard components for the addressee group “neonatal unit” apply to the place where preterm born and ill infants are cared for after birth until their discharge or in case of a rehospitalisation.
The addressee group “health service” includes several groups, such as professional healthcare societies, insurers, payers, and health system, depending on the national health system.
The components of the standards are categorised based on scientific evidence or practical expert experience for which a specific grading of evidence framework has been developed by the Chair Committee.
The system comprises three categories of evidence, each with different quality levels:
Category A refers to scientific evidence generated by systematic research on the basis of the GRADE approach. (1) As several components of individual standards are not based on scientific evidence but on shared cultural values or on best practice experiences, category B was introduced. Category C implies evidence from legal certainties such as laws, regulations, or court practice.
A. Scientific evidence is judged in terms of methodological flaws, consistency of results across studies, generalisability of research results, and effectiveness of treatments. Thus, it is assigned accordingly to a specific quality level, i.e. high, moderate, low, and very low. The four levels reflect the strength of a recommendation (1):
B. Levels of cultural values derived evidence are assessed by the geographic scope of these shared values as judged by the project’s experts:
C. Evidence from laws, regulations, or court practice is used as a third category. This type of evidence is also assessed based on the geographic scope of the legal source.
1Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924–6.
Quality measurement is very important in medical care, as without measuring there is no possibility to evaluate whether certain measures are implemented effectively and result in an improvement of care. In order to make the components of standards and implementation activities assessable, indicators for benchmarking and evaluation purposes are provided next to each component. However, due to the fact that these indicators need to be uniformly applicable, they were formulated broadly and need to be closely defined when adapting the standard to a national context. For example, the indicator ‘guideline’, can either mean that there is the need for a national or unit guideline or that the particular component is part of the guideline (e.g. definitions):
You are currently viewing a placeholder content from Facebook. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationYou are currently viewing a placeholder content from Instagram. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationYou are currently viewing a placeholder content from X. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More Information